PANCAIM
Overview
The central PANCAIM concept is to successfully exploit available genomic and clinical data to improve personalized medicine of pancreatic cancer. PANCAIM’s concept is unique as it integrates the whole spectrum of genomics with radiomics and pathomics, the three future pillars of personalized medicine. The integration of these three modalities is very challenging in the clinic, but also with AI. PANCAIM uses an explainable, data-efficient, two-staged AI approach. AI biomarkers transform the unimodal data domains into interpretable likelihoods of intermediate disease features. A second AI layer merges the biomarkers and responds with an integrated assessment of prognosis, prediction and monitoring of therapy response, to assist in clinical decision making. PANCAIM builds on four key concepts of AI in Healthcare: data providers, clinical expertise, AI developers, and MedTech companies to connect to data and bring AI to healthcare. Data quantity and quality is the main factor for successful AI. Partners provide eleven Pan European repositories of almost 6000 patients that are open to ongoing accrual. SME Collective Minds builds the GDPR data platform that hosts the data and provides a trustable connection to healthcare for even more and sustainable data. SME TheHyve builds tooling to connect to more genomic repositories (EOSC Health). Six Pan European academic centers provide clinical expertise across all modalities and help realize a curated, high quality annotated data set. Partners also include expert AI healthcare researchers across all clinical modalities with a proven track record. Finally, Siemens Healthineers provides their AI expertise and tooling to bring AI into healthcare for clinical validation and swift clinical integration in 3000 health care institutes.
Tasks
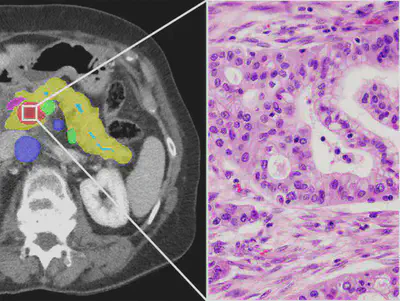
Pathology is a diagnostic gold standard for PDAC. However, the information it provides is limited to definitive confirmation of the diagnostic entity and the size and locoregional extent of the tumour. The only tumour-intrinsic feature is the grade of differentiation, a historical concept that is of no relevance for the management of PDAC. The rich information contained in tumour morphology integrates results of interactions at all levels - genetic, epigenetic, microenvironmental -, but is left totally unexplored. It is only with the advent of AI that attempts at deciphering this rich information have been undertaken. Recent studies on a variety of other cancers show that AI can extract information from routine pathology tissue sections that relate to underlying genomic aberrations and allows prognostic discrimination between patients that are currently lumped within the same clinical stage. With the wealth of data accessible in its repository, PANCAIM is in a unique position to decipher and fully exploit the prognostic and predictive information that hitherto has remained unmined in surgical and biopsy specimens from PDAC patients.
Recent studies on a variety of other cancers show that AI can extract information from routine pathology tissue sections that relates to underlying genomic aberrations and allows prognostic discrimination between patients that are currently lumped within the same clinical stage. Although this has been shown and applied in for example, breast cancer [Jaber et al, Breast Cancer Research, 2020] and mesothelioma [Courtiol et al, Nature Medicine 2019], these insights have not yet been applied to PDAC. A key reason is the limited availability of large datasets that combine radiology, pathology, genetics, and clinical follow-up. With the wealth of data accessible in its repository, PANCAIM is in a unique position to decipher and fully exploit the prognostic and predictive information that hitherto has remained unmined in surgical and biopsy specimens from PDAC patients. Specifically, it is our ambition to use novel machine learning techniques such as neural image compression [Tellez et al, TPAMI 2019] to elucidate key pathomics features which can help predict prognosis, treatment response and genetic alterations in PDAC (Figure 9, Figure 8). By additionally applying for the latest advances in explainable artificial intelligence, such as attention-weighting and saliency mapping, PANCAIM furthers the acceptance and integration of these pathomic features in clinical practice.